(stow-e-key-oh-metric)
Airplane engine chemistry, carbon monoxide detectors
and butter pecan ice cream.
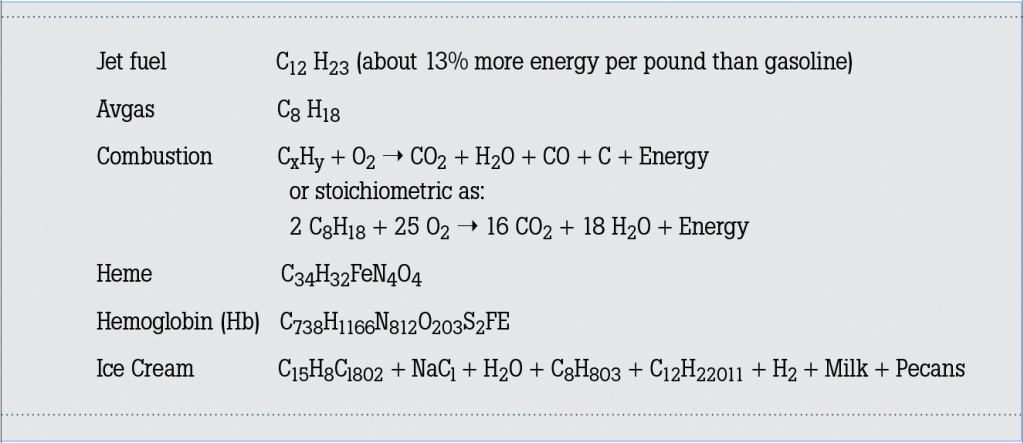
If you understand the relevance of the above compounds, chemicals, emulsions and reactions as they relate to flying fossil fuel-powered planes, then just go purchase a sensitive CO detector, put it in your airplane and move on to the next prodigious T &T article. If not, or if you simply can’t resist reading this humble column, then please continue. But be warned, even though this story may save your life, it’s going to taste more like insipid plain yogurt than tasty butter pecan ice cream.
Forward
In May of this year, I wrote an article titled “1.29 Gigawatts!” It was about electrical fires and onboard emergency equipment. I claimed that any old smoke/CO detector would be better than none at all and suggested a $36 detector from Home Depot. While this is obviously true, one of our longtime readers (a trained and qualified hazmat guy) pointed out that it’s not much more money, in airplane dollars, to get one that’s extra sensitive that will alert us sooner and thereby give us more time to get on the ground and into clean air. And thus was the impetus for this article. So, if you’re not a hazmat expert or versed in thermodynamics, engineering, chemistry, the respiratory system of vertebrates or in making ice cream, please read on.
Poison Contrails
An ideal fuel/air mixture in which both the fuel and the oxygen in the air are completely consumed is called the stoichiometric mixture and is
described as the ratio of the mass of air to the mass of a particular fuel. This ratio is approximately 14.7 to 1 for Avgas and about 14.5 to 1 for Jet A (jets vary significantly based on the specific engine). In the complete combustion of hydrocarbons (stoichiometric), the products are carbon dioxide, water and unaffected nitrogen. Notice how the CO from the first combustion equation above is missing from the second stoichiometric equation. With the incomplete combustion of hydrocarbons in recips and jets (the first equation), the products include unburned hydrocarbons, nitrogen oxides, carbon monoxide (the boogeyman in our story), carbon dioxide and water. These types of emissions are responsible for not only contrails but carbon monoxide poisoning. And there you have our story’s first spoonful of plain yogurt.
Why Not Stoichiometric?
Most recips and jets operate at something other than the stoichiometric mixture in order to achieve better economy, power or engine life. For example, an idling engine runs richer and colder than at cruise power, is even further from stoichiometric and produces a relatively high amount of CO. And if you operate LOP (Lean Of Peak) in your recip, you pass through the stoichiometric mixture on your way to LOP, and for a brief moment, you have an ideal fuel/air mixture and all CO is burned (at around 609 degrees Celsius). Once you get to LOP, combustion is once again cooler and the CO remains unburned. Running at the stoichiometric mixture is impractical because the mixture would burn too hot for engine longevity and power would be diminished. So, we are burning fuel at a ratio such that CO is produced and not burned. Now, here is a big spoonful of plain yogurt.


The Porphyrin Class
Carbon monoxide is a very important industrial compound used in refrigeration and cooling, as an inert gas in chemical processes, in the synthesis of ammonia, and in the storage of carbon powder in fire extinguishers. It burns in air with a bright blue, 2,121 degrees Celsius flame, is only slightly soluble inwater, and its physical properties closely resemble those of nitrogen. Carbon monoxide is a colorless, odorless and insipid (there’s that word again) gas and is the number one cause of accidental death from poisoning. The human respiratory system provides oxygen to the tissues and eliminates the carbon dioxide produced by them. When inhaled, carbon monoxide passes from our lungs into our bloodstream, where it attaches to the hemoglobin molecules that normally carry oxygen. Most of the O2 transported by the blood is through its reaction with hemoglobin molecules. Hemoglobin is a red protein responsible for transporting oxygen in the blood of all critters possessing an internal spine (that’s us). Its molecule is comprised of four subunits, each containing an iron atom bound to a heme group. Heme is an iron-containing compound of the porphyrin class (see formula #5 above), and makes our blood red and gives it the ability to carry oxygen. The transport of CO happens in the same way, but its attraction with hemoglobin is about 250 times greater than O2. Apparently, CO likes to stick to Hb even more than we like to eat butter pecan ice cream.
Neurological Conditions and Half-life
Without enough oxygen, individual cells suffocate and die, especially in vital organs such as the brain and heart. The most common symptoms of CO poisoning are headache, dizziness, weakness, upset stomach, vomiting, chest pain and confusion. Symptoms are often described as “flu-like.” If you breathe in a lot of CO it can make you pass out or kill you. If you are exposed to low levels of carbon monoxide over a longer period (by an exhaust leak, for example), your symptoms can also appear like the flu. The half-life of CO after a pilot resumes breathing “clean” ambient air is approximately 4 to 5 hours, while breathing high-flow oxygen via a non-rebreathing face mask (one that prevents rebreathing your own breath) is about 90 minutes, and with 100 percent hyperbaric oxygen (pressure breathing), approximately 30 minutes. An unusual feature of acute CO poisoning, however, is a delayed deterioration in neurological conditions occurring anytime from a few days to as long as five to six weeks after initial exposure.
What’s a Mother to Do?
CO poisoning from jet exhaust may be slightly less likely than from a recip, but any smoke or fumes would call for the same mayday call and emergency descent.
If you smell exhaust odors or begin to feel any of the symptoms previously mentioned, you should immediately assume carbon monoxide is present and take the following actions:
- Turn the cabin heat fully off.
- Increase the rate of cabin fresh air ventilation to the maximum.
- Open windows if the flight profile and aircraft’s operating manual permit such an action.
- If available (provided it does not represent a safety or fire hazard), consider using supplemental oxygen.
- Land as soon as possible.
- Do not hesitate to let Air Traffic Control know of your concerns. Use Mayday and ask for vectors to the nearest airport, request ARFF.
- Once on the ground, seek medical attention. Before continuing flight, have the aircraft inspected by a certified mechanic and consider CO’s half-life as described above as well as the delayed neurological issues.

CO Detectors; In My Own Words
Most retail brand carbon monoxide alarms are designed to meet minimum government standards (UL 2034 in the U.S. and CA 6.19 in Canada). These standards are meant to protect healthy adults from high levels of CO. Approved alarms must adhere to the following test point minimum
exposure times. Note: Carbon Monoxide is measured and displayed in “PPM” or parts per million.
- 0 – 29 PPM: The detector must remain silent. If it has a digital display, it must show a zero reading.
- 30 PPM – 69 PPM: If the carbon monoxide level remains in this range for a minimum of 30 days, the audible alarm may sound. If the unit has a digital display, it should display the CO level, provided it is 30 ppm or higher.
- 70 PPM – 149 PPM: The alarm must sound when levels reach this range for between 60 – 240 minutes.
- 150 PPM – 399 PPM: The alarm must sound if the carbon monoxide level remains in this range for between 10 to 50 minutes.
- 400 PPM +: The alarm must sound if the carbon monoxide level remains at or above this level for between 4 to 15 minutes.
Feel free to surf the web to select a CO detector of your own choosing and consider the following more sensitive alarm as an example: Available for about $190, CO Experts offers the Model 2016. It displays CO starting at 1 PPM and makes its first alert immediately with no time delay at 7 PPM. It has a graduated series of alarms as the CO concentration rises, and there is no time delay between hitting a concentration level and the alarm sounding. It has a silence feature allowing the user to shut off the alarm temporarily – the length of time the alarm will remain silent decreases at higher CO concentration levels, and the alarm will sound again after silencing if the CO level increases any amount. The unit has an expected life of five years and includes a monitoring system that warns of a detector failure, low battery and impending end of life of the unit.
CH4 Is Not A Noble Gas
(He, Ne, Ar, Kr, Xe, Rn and Og)
Thank you for reading my humble column, written left-handed from memory without the internet, while on a layover in BOS and not MIA (see “To Err Is Human,” T &T August 2020 for the relevance of MIA). You’ve made it through a life-saving, plain yogurt flavored article about CO, chemistry and our respiratory system and can now move on to the next T&T article without regret. Well, except for the regret you may feel after eating too much butter pecan ice cream. I say go for it; there are no ice cream detectors here. But make certain the ratio of ice cream to chocolate fudge is stoichiometric. If not, you may need to run yourself LOP to thwart N2 + H + CO2 + O2 + CH4 emissions. And if you’re in the yogurt business, please forgive me – no malice was intended; I just don’t like plain yogurt.

